Liver-on-chip: keeping up with the technology
Introduction to the liver-on-chip technology
Today’s issue
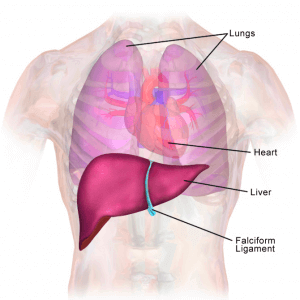
The liver is involved in more than 300 vital functions [1]. Still, it is mainly known for being part of the digestive tract, which has the critical role of metabolizing xenobiotics and nutrients (carbohydrates and lipids). In toxicological studies (either fundamental or during clinical trials for drug development), the chemical of interest is constantly tested on the liver.
Unfortunately, the predictions of the in-use models (in vitro or animal models) do not often correspond precisely to what is observed in humans. Liver toxicity (or so-called Drug-Induced Liver Injury, DILI) is one of the major causes that can halt the clinical phase in drug development, worsening the long delays of these drug tests [2].
The microfluidic device’s main challenge lies in the liver’s complexity. This organ is complex to model because of its numerous functions combined with a wide range of different kinds of cells and a specific architecture.
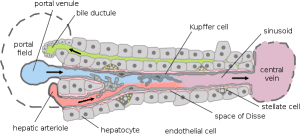
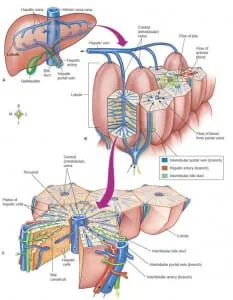
Among the different cell types, we find hepatocytes, Kupffer cells, and fibroblasts, which make the liver a versatile organ. Moreover, the other functions of this organ are organized following the phenomenon of hepatic zonation: liver cells have specialized functions based on their position along the portal vein to the central vein.
Different drugs are metabolized and cleared by cells in different zones. Adding a significant difficulty to the in vitro study of the liver, this phenomenon is highly associated with DILI. For all these reasons, a physiologically relevant liver model has emerged as an unmet need.
Hepatitis B's issue
Hepatitis B is a viral infection that attacks the liver and can cause acute and chronic diseases. An estimated 257 million individuals are infected worldwide, and over 750,000 people die of hepatitis B each year [3], mostly from complications like cirrhosis and hepatocellular carcinoma. An efficient vaccine is available, but there is no specific treatment for acute hepatitis B. As this disease only affects humans and chimpanzees, developing a therapy has an additional limitation. A liver-on-chip would accelerate this research.
Previous solutions
A lot of in-vitro model systems have already been developed for the liver. Their primary purpose was to investigate the potential adverse effects of chemicals and drugs. Liver tissue slices, perfused liver, and mainly immortalized cell lines and isolated liver cells [4] have been used. Despite a continuous increase in the application of these conventional in vitro models, they have not been satisfactory in entirely replacing animal models to predict human toxicity.
They present a lot of problematic limitations, mainly a loss of viability, a limited throughput, and a decrease in liver-specific functionalities. To prevent those, cocultures of various cell types with hepatocytes (to avoid de-specializing the cells) and three-dimensional tissue constructs and bioartificial livers are investigated.
Despite these developments, most technologies fail to mimic the multifaceted physiology of the liver in long-term culture models, especially regarding the acetaminophen (APAP)-induced hepatotoxicity and the relevant zonal effects in the liver [4].
The liver-on-chip solution
This architecture also helps the cells to live longer. Consequently, it allows for more extended studies, which can be significant, especially in identifying side effects that take longer to appear [5].
Technical characteristics of the liver-on-chip
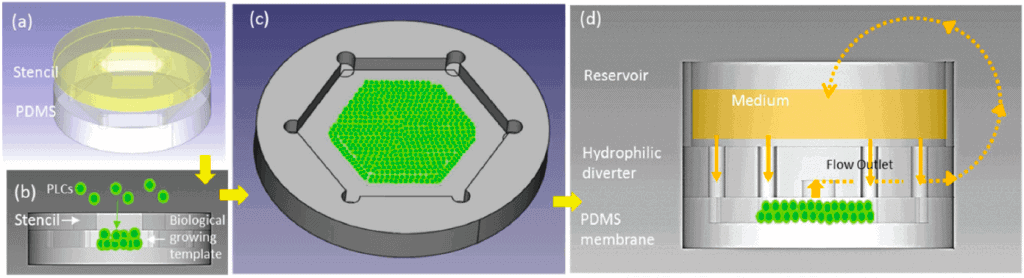
The device consisted of a micro-patterned hydrophobic polydimethylsiloxane (PDMS) membrane with a depth of 150 µm that was fabricated for multilayered cell depositions.
The cells deposited were primary liver cells, forming a biological growing template on the collagen-coated PDMS membrane. The cell-loaded PDMS membrane was enclosed to create a culture chamber with a hydrophilic flow diverter to ensure a vertical cell anchorage.
A peristaltic pump in the medium between the reservoir and the liver-on-chip mimicked the circulation between the liver and the body. The culture chamber was hexagonal to simulate the portal vein function, and the inlets were located at each corner of the chamber.
A flow was introduced radially from six discrete inlets into the culture chamber. To mimic the flow from the portal vein to the central vein, an outlet was positioned in the center of the culture, which received the flow from the inlets. The flow may pass through the structure corresponding to the hepatic cord radially, which can simulate biomimetic radial flow in the liver lobule. With the help of a scanning electron microscope (SEM), they further investigated the progressive morphogenesis of the LOC.
Study of multiple polarities of the liver-on-chip
To further understand the development of multiple polarities in the liver-on-a-chip, they used MRP2 to investigate functional polarization and F-actin for structural polarization. MRP2 is a liver transporter located on polarized apical canaliculi between hepatic cords and is responsible for drug excretion. MRP2 dysfunctions are associated with drug resistance and DILI [7, 8]. For its part, F-actin is a cortex cytoskeleton that seemed ideal for studying the dynamics of cell remodeling and assembly during organogenesis [9, 10, 11].
Results obtained with the liver-on-chip
Global viability and organotypic architecture
After a week, the liver-on-chip culture showed good cell viability. The analyses of the different images of the culture revealed the hepatic cords-like architectures in the liver-on-chip on day 7. The features observed resembled the lobule of a living liver.
With three days of perfusion, the primary liver cells were formed into round-shaper clusters on the growing template, and the flow removed some unhealthy cells, creating multiple cell clusters amid space in the device.
These clusters were gradually connected, assembled, and organized into an organotypic architecture in one week. This could be identified as the typical sinusoid wall-like morphology and the fenestrated window-like nanostructure.
Moreover, for the MRP2 test, after one week of the liver-on-chip culture, the distribution of MRP2 was optically reconstructed and resembled a liver tubule. MRP2 was expressed in each cell and was also polarized, connected, and reassembled into a nanonetwork across an apical domain of connected hepatocyte cords.
On day 3, the F-actin was expressed and polarized toward a junction of cell contacts and cell cortex, meaning there was cell-cell contact and membrane integration. F-actin polarized to the peripheral cortex of cells and developed a 3D intracellular skeletal network resembling a liver lobule. In the control culture, the F-actin expression faded after the first week.
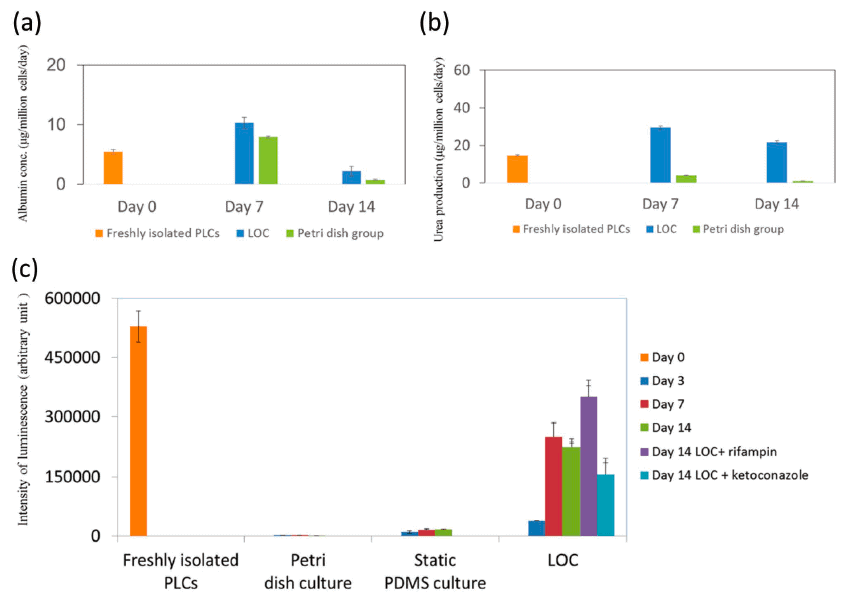
Liver function study
After one and two weeks, they decided to evaluate the albumin and urea synthesis of the liver-on-chip as feedback concerning the state of the liver’s specific functions.
These two proteins are synthesized in the liver, indicating their activation level. In the liver-on-chip group, both albumin and urea synthesis were restored after one week. After two weeks, these concentrations slightly decreased but remained significantly higher than those observed in the control culture (conventional Petri dish culture).
Drug metabolism study
To evaluate the drug metabolism capacity and the device’s clearance, they decided to quantify the activity of cytochrome P450 3A4 (CYP 3A4). CYP 3A4 is one of the most important enzymes involved in the metabolism of xenobiotics. They found that its activity was successfully maintained for weeks in the liver-on-chip and was a lot higher than the one observed in the control cultures (both Petri dish culture and static PDMS culture).
They also evaluate the metabolic dynamics of the device by applying rifampin and ketoconazole as inducers and inhibitors of CYP, respectively [12]. After 12h of dosing, on day 14, the metabolic activity of the liver-on-chip was increased in the rifampin group and reduced in the ketoconazole group.
Further applications of the liver-on-chip device
Conclusion of the research
Moreover, although the device helped achieve better results, it seems that the liver-specific activities were decreasing after two weeks, maybe meaning there was a loss of specialization. Thereby, some improvements can be made regarding the coculture, in which we could add other cells involved in the liver, like Kupffer cells. The primary hepatic stellate cells were isolated from rats.
This also represents a limitation to this paper since we would further want to use this device for diseases that sometimes affect only humans and chimpanzees (like hepatitis B). From a global view, replacing these cells with human hepatocytes would allow for more correct studies. Another way of improvement would be to recreate the bile ducts, which have not been investigated yet despite their central position in the liver.
Further research
Another promising ongoing research was stated by Khazali et al. [15], who used liver-on-chip platforms to target liver metastases to improve patient outcomes.
Finally, the idea of connected liver-on-chip and gut-on-a-chip would provide a promising technology, giving a more global vision of how a xenobiotic is washed out of the body (first-pass effect).


Authors:
Emilie Grandfils, Julie Cavallasca and
Guilhem Velvé Casquillas
With the support of the NMBP-RIA PANBioRA project
Grant agreement number: 760921
Contact:

References
- Natalie Angier, “Physiologie. Le foie, cet organe à tout faire”, Courrier international (The New York Times), 13 juillet 2017
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739.
- Hepatitis B Fact sheet N°204. who.int. July 2014. Retrieved 4 November 2014
- Toxicol Res (Camb). 2013 Jan 1;2(1):23-39. Epub 2012 Nov 23. In vitro models for liver toxicity testing. Soldatow VY, Lecluyse EL, Griffith LG, Rusyn I.
- “the cure – liver on a chip”, https://www.youtube.com/watch?v=2EJlRXvpnf8
- Weng YS, Chang SF, Shih MC, Tseng SH, Lai CH, 2017 Jul 21. doi: 10.1002/adma.201701545. Scaffold-Free Liver-On-A-Chip with Multiscale Organotypic Cultures.
- M. Trauner, J. L. Boyer, Physiol. Rev. 2003, 83, 633
- A. Esteller. World J. Gastroenterol. 2008, 14, 5641
- E. M. Huisman, T. V. Dillen, P. R. Onck, E. V. Giessen, Phys. Rev. Lett. 2007, 99, 208,103
- L. Lanzetti, Curr. Opin. Cell Biol. 2007, 19, 453.
- J. D. Humphrey, E. R. Dufresne, M. A. Schwartz, Nat. Rev. Mol. Cell Biol. 2015, 15, 802.
- E. L. LeCluyse, R. P. Witek, M. E. Andersen, M. J. Powers, Crit. Rev. Toxicol. 2012, 42, 501.
- Rinella ME, June 2015, Nonalcoholic fatty liver disease: a systematic review.
- Manuele Gori, Maria Chiara Simonelli, Sara Maria Giannitelli, Luca Businaro, Marcella Trombetta, and Alberto Rainer, 2016, Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device
- A. S. Khazali, A. M. Clark, A. Wells, June 2017, A Pathway to Personalizing Therapy for Metastases Using Liver-on-a-Chip Platforms
- Written by Emilie Grandfils, corrected by Julie Cavallasca, under the supervision of Dr. Guilhem Velve Casquillas