Drug testing: organ-on-chip models vs. standard models
Introduction to organ-on-chip models
In vitro and in vivo models are widely used to investigate human pathophysiology and toxicology studies. The goal of this review is to highlight the main advantages and limitations of each model and to show how the use of organ-on-chip model technology can address their drawbacks.

The article refers specifically to lung systems (i.e., capillary-alveolar interface). Still, it also applies to other organs since the characteristics of joint in vivo/ in vitro systems result in being related to the system itself rather than the organ/ tissue studied.
You can look here for a description of the use of the alveolar-capillary barrier to design microfluidic lung-on-a-chip systems. To start using microfluidics to build lung-on-a-chip models, check out our pack.
You can continue reading here to learn more about the origin and design of lung-on-a-chip systems.
Overview of organ-on-chip in vitro and in vivo models
Organ-on-chip models vs. ANIMAL MODELS [1, 2]
| |
+ |
|
– |
|
Organ-on-chip models vs. EX VIVO CULTURES (e.g., biopsy samples) [3, 4]
| |
+ |
|
– |
|
Organ-on-chip models vs. 2D CELL CULTURES [3, 4]
| |
+ |
|
– |
|
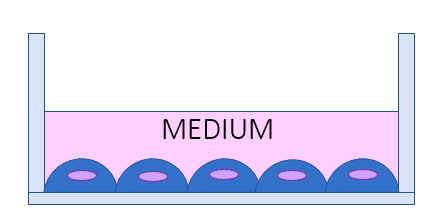
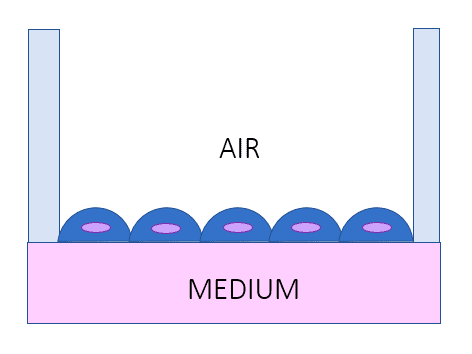
Organ-on-chip models vs. AIR-LIQUID INTERFACE LUNG MODEL [1, 5]
| |
+ |
|
– |
|
Organ-on-chip models vs. ORGANOIDS [6, 7]
| |
+ |
|
– |
|
Organ-on-chip models vs. TISSUE ENGINEERING (1)
Artificial constructs [8-10] | |
+ |
|
– |
|
Organ-on-chip models vs. TISSUE ENGINEERING (2)
Biological (decellularized) constructs [8–10] | |
+ |
|
– |
|
Organ-on-chip models as solutions for the limitations of traditional in vitro and in vivo systems
Advantages resulting from the fabrication process
- Modularity and variety of techniques/ materials for microfabrication
- The chip design can be easily created and modified based on need
- High reproducibility of fabrication protocols
- Possibility to integrate sensors for high-throughput analyses
- Possibility to develop personalized models
Advantages resulting from a microscale flow
- Mimic physiological/diseased mechanical cues at a cellular/ tissue level
- Create a controlled and dynamic microenvironment
- Recreate the air-liquid interface (specifically for lungs)
Advantages for the cellular/ biological component
- Create a complex tissue-like environment with several cell types
- Create modular devices mimicking different organ areas (ex, bronchi, alveoli, etc.…in the case of lungs)
- Reproducing physiological and pathological conditions
Advantages for time/cost of research
- Fast fabrication processes (soft lithography, rapid prototyping, …)
- Possibility to rapidly test drugs/compounds and have high-throughput outcomes.
- Possibility to easily mimic disease conditions without the need to prepare, maintain, and use animal models.

Review done thanks to the support of the DELIVER H2020-MSCA-ITN-2017-Action “Innovative Training Networks.”
Grant agreement number: 766181
Written by Alessandra Dellaquila, PhD
Contact:

References
- A. J. Miller and J. R. Spence, “In vitro models to study human lung development, disease and homeostasis,” Physiology, vol. 32, no. 3, pp. 246–260, 2017.
- B. A. Hassell et al., “Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro,” Cell Rep., vol. 21, no. 2, pp. 508–516, 2017.
- C. Blume and D. E. Davies, “In vitro and ex vivo models of human asthma,” Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV, vol. 84, no. 2, pp. 394–400, Jun. 2013.
- H. Behrsing et al., “Assessment of in vitro COPD models for tobacco regulatory science: Workshop proceedings, conclusions and paths forward for in vitro model use,” Altern Lab Anim, vol. 44, no. 2, pp. 129–166, 2016.
- R. Bhowmick and H. Gappa-Fahlenkamp, “Cells and Culture Systems Used to Model the Small Airway Epithelium,” Lung, vol. 194, no. 3, pp. 419–428, Jun. 2016.
- K. Gkatzis, S. Taghizadeh, D. Huh, D. Y. R. Stainier, and S. Bellusci, “Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease,” Eur. Respir. J., vol. 52, no. 5, Nov. 2018.
- M. Huch, J. A. Knoblich, M. P. Lutolf, and A. Martinez-Arias, “The hope and the hype of organoid research,” Development, vol. 144, no. 6, pp. 938–941, Mar. 2017.
- A. Doryab, G. Amoabediny, and A. Salehi-Najafabadi, “Advances in pulmonary therapy and drug development: Lung tissue engineering to lung-on-a-chip,” Biotechnol. Adv., vol. 34, no. 5, pp. 588–596.
- R. Langer and J. Vacanti, “Advances in tissue engineering,” J. Pediatr. Surg., vol. 51, no. 1, pp. 8–12, Jan. 2016.
- D. M. Hoganson, E. K. Bassett, and J. P. Vacanti, “Lung tissue engineering,” Front. Biosci. Landmark Ed., vol. 19, pp. 1227–1239, Jun. 2014.